Nursing Update on New COPD Inhaler Devices – Part 2 : Ellipta®
Ellipta® Inhaler Update
The Ellipta® (DPI-dry powder inhaler ) is currently in use to deliver 3 recently approved medications; Breo®(fluticasone/vilanterol), Anoro®(umeclidinium and vilanterol), and Incruse®(umeclidinium). Breo® is a combination steroid and long acting beta-agonist used to manage Asthma or COPD, Anoro® is a combination long-acting muscarnic and long-acting beta-agonist to manage COPD, and Incruse® is a long-acting muscarnic to manage COPD.
Administration
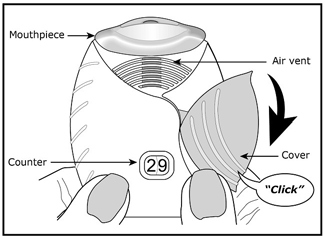
- Slide cover to expose mouthpiece. Listen for a click and ensure the counter is decreased by one.
- Instruct the resident to breathe out (away from the Ellipta inhaler).
- Place lips around the mouthpiece inhaling quickly (be careful not to block air vents during inhalation).
- Instruct the resident to hold their breath for 4 seconds and breathe out slowly.
- Close the inhaler and ensure resident rinses their mouth after Breo® use as it contains the steroid fluticasone. Failure to rinse will increase the risk of oral infections such as thrush.
Storage and Labeling: Store the inhaler at room temperature in the tray or packaging provided by the pharmacy. Write the date opened and discard date on the inhaler label. The discard or expiration date is 6 weeks from the date you open the tray and remove the inhaler.
***Important*** If you open and close the cover without inhaling the medicine, you will lose the dose. The lost dose will be held in the inhaler, but it will no longer be available to be inhaled. Thus, it is not possible to accidentally take a double dose or an extra dose with one inhalation.
References:
https://www.gsksource.com/pharma/content/gsk/source/us/en.html