Nursing Update on New COPD Inhaler Devices – Part 3 Pressair® Inhalers
Pressair® Inhaler Update
The Pressair® inhaler device contains the medication aclidinium bromide(Tudorza®), an anticholinergic medication for the treatment of chronic obstructive pulmonary disease (COPD). It is recommended to be administered by inhalation twice daily.
Administration
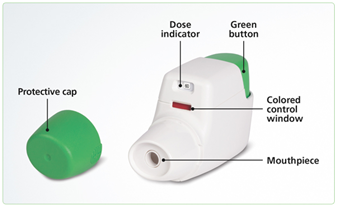
- Remove the protective cap.
- Press the green button all the way down and release getting a dose
- ready (do not inhale at this time).
- Check the control window. If it is green the dose is ready, if it is red repeat step 2.
- Instruct the resident to breathe out and place lips on the mouthpiece, then to breathe in quickly until you hear a “click” sound. The dose is not fully administered if you do not here a click. Do not hold or press the green button during inhalation.
- Tell the resident to hold their breath for as long as possible, breathing slowly out through their nose when finished.
- Check the control window again and ensure it has turned red (this is the clicking sound you heard during inhalation). If the window is green repeat steps 4 and 5 again. If the resident cannot inhale properly, communicate to their physician or your AlixaRx Clinical Pharmacist for alternatives.
- Place the protective cap back on the inhaler and store in the medication cart.
Storage and Labeling: Store the inhaler at room temperature in packaging provided from the pharmacy. Write the date opened and discard date on the inhaler label. The discard or expiration date is 45 days from the date the sealed pouch was opened. Discard the Pressair® inhaler after 45 days, when the dose indicator reads zero, or when the inhaler locks out and the green button can no longer be depressed, whichever comes first.
References: 1. http://www.tudorza.com